how many valence electrons are in alkali metals|How many valence electrons are in alkali metals? : Tagatay Mar 23, 2023 MANILA, Philippines – The O-P passers in the October 2022 Licensure Exam for Teachers (LET) Secondary Level are available here as released by PRC fifty-six (56) working days after the board exams. The Licensure Exam for Teachers was conducted on October 2, 2022 at several testing centers located at Manila, Antique, Bacolod, .
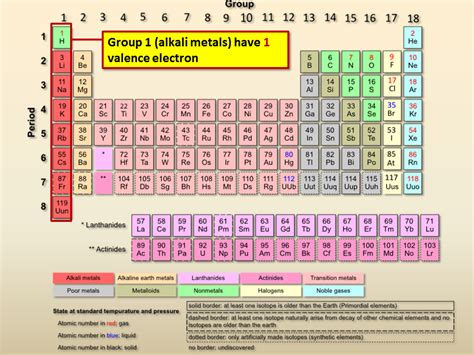
how many valence electrons are in alkali metals,All alkali metals ( belongs to group 1 of periodic table) have 1 valence electron. Valence electron are those electron that are present in the outermost orbit of the atom. Alkali metal present in .

Mar 23, 2023
Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur (S) is located in Group VIA (Group 16), . There are two ways to find the number of valence electrons in the Alkali Metals. The first is to use the Periodic Table to figure out how many electrons Alkali Metals have in .
how many valence electrons are in alkali metals How many valence electrons are in alkali metals? In addition to solvated electrons, solutions of alkali metals in liquid ammonia contain the metal cation (M +), the neutral metal atom (M), metal dimers (M 2), and the metal anion (M −). The anion is formed by adding an electron to the .
Alkali metals have one electron in their valence shell. The electronic configuration is given by ns 1 . For example, the electronic configuration of lithium is given by 1ns 1 2ns 1. They tend to lose the outer shell electron to form . How many valence electrons do alkali metals have? Alkali metals have only one electron in their Valence shell (ns ¹ ). Why is hydrogen grouped with alkali metals? All atoms naturally want to have full set of outer electrons. However, elements in that first column of the periodic table all have one electron in their outermost shell. This outer shell is also called the valence shell, and .
With the exception of groups 3–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of .
The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron–electron repulsions are reduced. The entering electron does not experience as much repulsion and the chlorine atom accepts an additional .Although many of these properties are similar to those of the alkali metals (Table \(\PageIndex{1}\)), certain key differences are attributable to the differences in the valence electron configurations of the two groups (ns 2 for the . How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. . 8 valence electrons; Answer A. . How Many Valence Electrons Are in Group 1A? The defining characteristic of the alkali metals is the reactivity that they have towards water. This reactivity is directly related to having only one . For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
There are two ways to find the number of valence electrons in the Alkali Metals. The first is to use the Periodic Table to figure out how many electrons Alka.Alkaline Earth Metals. Group 2 elements are referred to as “alkaline earth” metals (tan column below).The name “alkaline” comes from the fact that compounds of these elements form basic (pH greater than 7) or alkaline solutions when dissolved in water.If the Group 1 elements all have one \(s\) electron in their outer orbital, we can predict that the Group 2 elements will have two .
This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons.The f-subshell holds 14 electrons and the g-subshell contains up to 18 electrons.Metals in the middle of the periodic table become more stable by emptying a shell, half-filling it, or completely filling it.
How many valence electrons are in alkali metals? Elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table.. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell with a noble gas electron configuration ending in #ns^2 np^6#.. METALS. The most reactive metals are those from Groups 1 and 2. Although many of these properties are similar to those of the alkali metals (Table 20.3.1), certain key differences are attributable to the differences in the valence electron configurations of the two groups (ns 2 for the alkaline earth metals versus ns 1 .how many valence electrons are in alkali metalsElectronic Configuration of Alkali Metals. Alkali metals have one electron in their valence shell.; The electronic configuration is given by ns 1.. For example, the electronic configuration of lithium is given by 1ns 1 2ns 1.; They tend to .This category includes all the nonmetallic elements, as well as many metals and the metalloids. The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 .

- Alkali metals have one electron in the s-orbital and their valence shell outside the noble gas core and the general electronic configuration of these alkali metals are represented as, $[Noble-gas]n{{s}^{1}}$ Therefore, they have only 1 electron in their outermost orbit. Thus, the correct answer is alkali metals have 1 valence electron. Note . Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
Alkali Metals: Group 1 (IA) - one valence electron; Alkaline Earth Metals: Group 2 (IIA) - two valence electrons; Transition Metals: Groups 3-12 - two valence electrons; Boron Group or Earth Metals: Group 13 (IIIA) - three valence electrons; Carbon Group or Tetrels: Group 14 (IVA) - four valence electrons;
Phosphorus has 5 valence electrons 2 from the 3s and 3 from the 3p. Lets take the ionic formula for Calcium Chloride is #CaCl_2# Calcium is an Alkaline Earth Metal in the second column of the periodic table. This means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. This makes .2) The number of valence electrons present in the alkaline earth metals is 2 . They lose two electrons to form M + 2 ions. For example, Magnesium (Mg) has an electronic configuration 1 s 2 2 s 2 2 p 6 3 s 2. After losing one electron, the electronic configuration will be 1 s 2 2 s 2 2 p 6. Hence, valence electrons are present in the alkali . How Many Valence Electrons to Alkaline Earth Metals Have? Valence electrons refer to the number of electrons found in the outermost shell of an atom. Alkaline earth metals are characterized by .
how many valence electrons are in alkali metals|How many valence electrons are in alkali metals?
PH0 · Valence Electrons Chart for All Elements
PH1 · How to Find the Number of Valence Electrons for Alkali Metals
PH2 · How many valence electrons do Alkali metals have?
PH3 · How many valence electrons are in alkali metals?
PH4 · Alkali metals: Definition, Properties and facts
PH5 · Alkali Metals: Elements in the First Column of the Periodic Table
PH6 · Alkali Metals: Elements in the First Column of the
PH7 · Alkali Metals
PH8 · 3.8: Electron Configurations and the Periodic Table
PH9 · 20.4: The Alkali Metals (Group 1)
PH10 · 10.6: Valence Electrons